Last spring, the innovative formulators at CV Sciences launched Relief, an ingenious way to augment the pain-relieving properties of CBD. It was so popular that they sold out within months! Now, Relief is back in stock and you can once again take advantage of its unique properties. Get a glimpse of the science here.
And don’t forget—my audience can get 30% off when you use code HOFFMAN30 at checkout.
—Dr. Ronald Hoffman
This article contains content from one of our trusted sponsors.
Occasional aches and pains are a common human experience, as we all feel the effects of physical over-exertion, minor injuries, increasingly sedentary lifestyles, and aging bodies. This causes many of us to reach for traditional pain medications almost daily. However, awareness of the negative impact that long-term use of OTC pain killers has on our bodies is increasing, leading pain sufferers to search elsewhere for safe and effective alternatives.
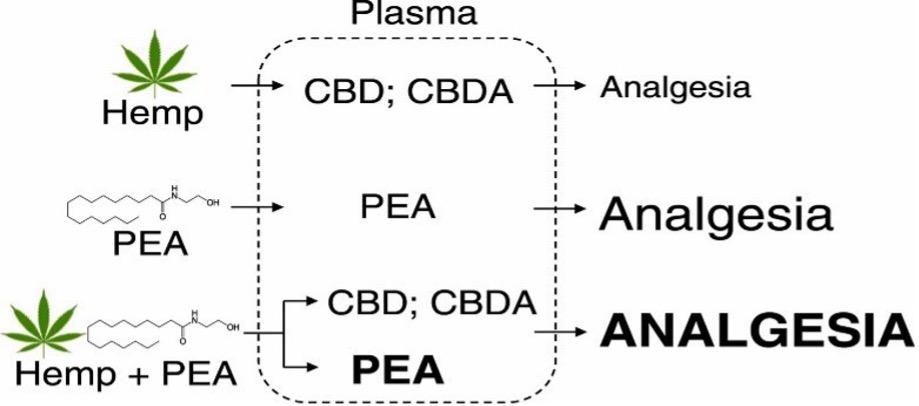
New on the shelves is +PlusCBD Relief for anyone experiencing the minor aches and pains and low-grade chronic inflammation that seems to drive all health issues. +PlusCBD Relief offers the first and only triple-action combination of CBD, CBDA, and PEA (palmitoylethanolamide) available to consumers. This powerful trio is more effective when used together in the Relief formula than just taking CBD, CBDA, or PEA alone. Let’s take a look at these three powerful ingredients and how they contribute to greater soreness support.
Cannabidiol (CBD)
The major cannabinoid found in hemp, CBD interacts with the body’s endocannabinoid system to support physical, mental, and emotional balance, helping maintain a healthy inflammatory response and regulating pain sensation with modulatory actions at all stages of pain processing by the body. CBD also plays a role in regulating innate immunity and inflammatory responses through the inhibition of pro-inflammatory cytokines and upregulation of anti-inflammatory cytokines. +PlusCBD Relief uses CO2 extracted full spectrum hemp that has been distilled for maximum strength potency. +PlusCBD is backed by more science than any other CBD brand available without a prescription.
Cannabidiolic Acid (CBDA)
CBDA is the acid-bound form of CBD, and unlike commonly used ethanol or butane extraction, the patented water-based extraction method used by +PlusCBD for its CBDA does not use heat or harsh chemicals to produce the purest source of CBDA. This water extracted CBDA is known to have increased bioavailability and offers its own set of unique physiological benefits. CBDA helps support a healthy inflammatory response by selectively modulating the COX2 enzyme pathway and helps promote a healthy gut.
Levagen® + (PEA)
PEA, or palmitoylethanolamide, is an endocannabinoid-like compound made by the human body. Relief softgels feature patented Levagen®+ PEA, which has been shown in human clinical studies to support a healthy immune, inflammatory, neurological, and nerve function response.
High-stress lifestyles and poor nutrition can lead to a deficiency of PEA. As a daily supplement, Levagen®+ PEA provides many of the same benefits as CBD and has been shown clinically to be more effective at reducing pain than common non-steroidal anti-inflammatory drugs (NSAIDs) like Ibuprofen. PEA is a broad modulator of the inflammatory processes that affect pain sensation and neuroprotection. Its mode of action has multiple effects (i.e., pleiotropic), as it involves many pathways and targets in both the peripheral and central nervous systems.
2,500 patients have been treated with PEA for various pain conditions in more than 30 clinical studies. Fifteen of these studies were randomized, controlled trials (RCTs) with a total of approximately 1,500 patients where PEA consistently demonstrated statistically significant reductions in pain with favorable safety and tolerability. The largest study published so far was a multicenter, double-blind randomized study on three groups (placebo, 300, and 600 mg PEA) of 636 total patients with a treatment duration of 3 weeks. These patients suffered low back pain/sciatica, and PEA was found to be efficacious and extremely well-tolerated.
The authors reported a Number Needed to Treat (NNT) for ≥50% pain relief was much lower than the treatments commonly used. Number to Treat (NNT) is specific to an outcome, which in this case is ≥50% Pain relief in lumbosciatic algias with only 1.7 people needing to take 600 mg of PEA (343 mg Levagen®+ PEA) daily for 1 person to experience ≥50% pain relief from low back pain due to compression of the sciatic nerve. PEA has no overt toxicity, even at high doses, and clinical trials have reported that the compound is very well tolerated, making it ideal for daily use.
Relief softgels are more effective than using CBD, CBDA, or PEA alone.
+PlusCBD Relief is powered by three of the most effective, non-habit-forming anti-inflammatories available — CBDA, CBD, and PEA — offering a safe alternative to risky non-steroidal anti-inflammatories and dangerous opioid pain killers. +PlusCBD Relief softgels support normal levels of your body’s natural anti-inflammatory and analgesic agents for maximum comfort and systemic relief.
Stay up to date on the research on CBD, cannabis & the cannabinoids as they relate to your quality of life: YOUR CANNABINOID RESEARCH LIBRARY
Stuart Tomc is the former Vice President of Science, Regulation, and Education for CV Sciences, Makers of PlusCBD Oil.
References
- Palmitoylethanolamide and hemp oil extract exert synergistic anti-nociceptive effects in mouse models of acute and chronic pain. Pharmacological Research (2021)
- Petrosino S., Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. J. Pharmacol.2017;174:1349–1365
- Petrosino and Moriello. Palmitoylethanolamide: A Nutritional Approach to Keep Neuroinflammation within Physiological Boundaries – A Systematic Review. J Mol Sci. 2020;21(24):9526.
- D’Amico et al. Palmitoylethanolamide and Its Formulations on Management of Peripheral Neuropathic Pain. Int J Mol Sci. 2020;21(15):5330
- Artukoglu et al. Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis. Pain Physician.2017 Jul;20(5):353-362
- Paladini et al. Palmitoylethanolamide, a Special Food for Medical Purposes, in the Treatment of Chronic Pain: A Pooled Data Meta-analysis. Pain Physician.2016 Feb;19(2):11-24
- Cruccu et al. Micronized Palmitoylethanolamide: A Post Hoc Analysis of a Controlled Study in Patients with Low Back Pain – Sciatica. CNS & Neurological Disorders – Drug Targets,2019,18:491-495.
- Guida et al. Palmitoylethanolamide (Normast) in chronic neuropathic pain caused by compressive-type lumbar sciatica: a multicenter clinical trial. DOLOR. 2010;25:35-42.
- Steels et al. A double-blind randomized placebo controlled study assessing safety, tolerability and efficacy of palmitoylethanolamide for symptoms of knee osteoarthritis. Inflammopharmacology. 2019;27(3):475-485.
- Cocito et al. Short-term efficacy of ultramicronized palmitoylethanolamide in peripheral neuropathic pain. Pain Res Treat. 2014;2014:854560.
- Schifilliti et al. Micronized Palmitoylethanolamide Reduces the Symptoms of Neuropathic Pain in Diabetic Patients. Pain Res Treat. 2014;2014:849623.
- Gatti et al. Palmitoylethanolamide in the treatment of chronic pain caused by different etiopathogenesis. Pain Med. 2012;13(9):1121-30
- Schifilliti et al. Micronized Palmitoylethanolamide Reduces the Symptoms of Neuropathic Pain in Diabetic Patients. Pain Res Treat. 2014;2014:849623.
- Gatti et al. Palmitoylethanolamide in the treatment of chronic pain caused by different etiopathogenesis. Pain Med. 2012;13(9):1121-30.
- DRG, Unipolar Depression Disease Landscape & Forecast. Sept 2020
- DRG, Epilepsy Disease Landscape & Forecast. Jan 2021
- Epilepsy Foundation
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7662788/pdf/ijms-21-07942.pdf







